Research Development Unit (RDU)
The RDU team provides research oversight, advice, training and support to staff and students across all Barwon Health locations. Our role is to ensure the protection of research participants and that all research activities under the auspices of Barwon Health meet the highest standards of research ethics and governance. The RDU supports staff and students to design and implement high quality research that improves the health and wellbeing of the community.
RDU is here to help.
If you are not sure where to start or have a question regarding your research project, email us at This email address is being protected from spambots. You need JavaScript enabled to view it. and a member of the RDU team will get back to you within 24 hours. If you have a current application underway, please ensure you include your Barwon Health reference number in your enquiry.
Meet the RDU team here.
Find out about the Research Ethics and Governance changes under new updated NHRMC National Statement on Ethical Conduct in Human Research (July 2023) here.
Contact Us
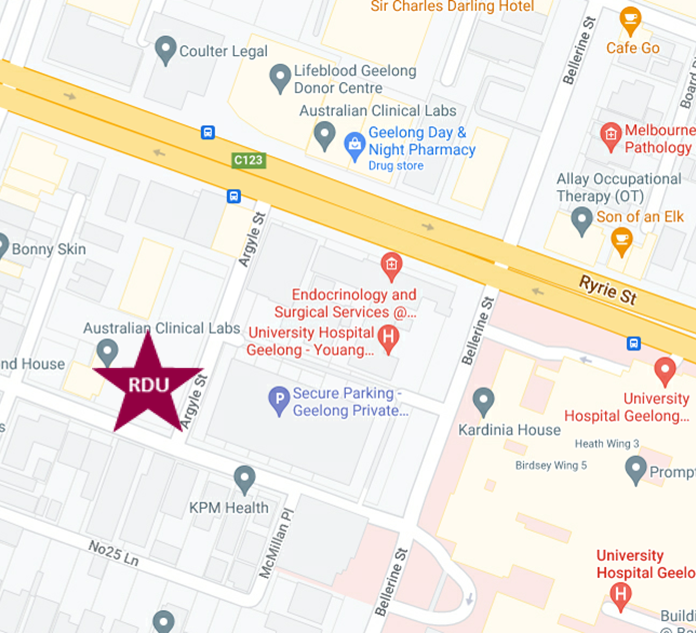
RDU Offices
Adrian Costa Clinical Trials Centre
Level 2, 73 Little Ryrie St
Geelong VIC 3220
Phone: (03) 4215 3374
Email: [email protected]