Introduction to Clinical Trial Research
The purpose of this page is to provide information, resources and tools to introduce you to clinical trial research and support you through the process of conducting clinical trial research.
1. Overview of Clinical Trial Research Process
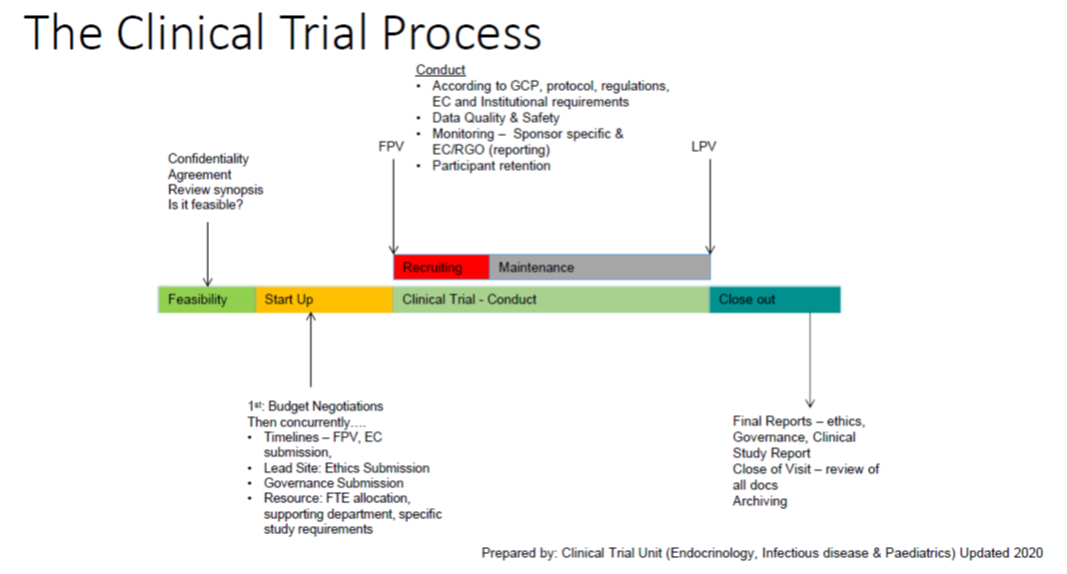
The Clinical Trial Process can be divided into the following stages:
- Feasibility
- Start-up
- Conduct
- Close-out
1.1 Feasibility
This stage refers to understanding whether you can do a study. If you have been offered the opportunity to provide feedback on a commercially sponsored trial then you will be sent a Confidentiality and Disclosure Agreement (CDA) to sign before you can review any study related information. Once returned, the protocol or a protocol synopsis will be provided to you to review. To understand the feasibility of a trial you need to consider the following:
- Do we have access to the study population described in the inclusion & exclusion criteria in the protocol? If so, how do they present to us and how will we recruit them?
- How many of these participants do we have access to? At what frequency?
- How many are likely to participate and over what period of time?
- Are procedures in the study design standard care or additional to standard of care? If so, what are they and will they collect valuable information to support the outcomes of the trial?
- What information is provided about the Investigational Medicinal Product (IMP), device or intervention? Do we know enough about the IMP (i.e. have your also been given and Investigator's Brochure, Product Information or Summary of Product Characteristics to review)? Is it addressing an unmet need?
- What other clinical trials have been conducted to date? What is the safety profile and how it is metabolised? Are there any concerns?
When the Principal Investigator and research team have evaluated the study they will respond back to the sponsor, with their level of interest in the study and potential numbers to contribute to the global or national trial.
The sponsor may request further information, as well as a Pre-Study Visit (PSV) or Site Selection Visit (SSV). These are half day visits where the sponsor meets the local research team, reviews facilities and discusses experience and expectations, to determine if they are an acceptable site to conduct the trial.
It is also an opportunity for the research team to discuss the protocol, learn more about the intervention, discuss ethics, governance, training, budgets and any perceived issues or concerns about going ahead with the trial.
By the end of the visit, the sponsor will know if the site is a 'preferred site' to conduct the trial. The trial site will also know if they would like to be involved.
1.2 Start-up
Once selected as a site, the clinical trial process moves into the Start-Up phase. In this phase there are several process occurring in parallel, however it is defined by:
- Developing a plan for the clinical trial, which includes understanding the following dates and milestones: final protocol, ethics and governance approval, first patient visit (FPV), recruitment start date and period, last patient visit (LPV), close out.
- Budget and Contract negotiations, including trial resource allocation by the site.
- Ethics: Whether you are lead site or a participating site
- Governance: Ensuring all supporting departments have been consulted, and any fees or particular needs for them to support the trial are included in the budget.
It’s important to appreciate that start up means a site has agreed in principle to conduct the trial, but organisational authorisation is not given until Research Governance Authorisation has been provided. Therefore, start-up is also defined by evaluating the ability of the site to conduct trial at every step of the start-up process, appreciating that as more information is provided about a trial it may not be feasible. For example, the timelines may be unrealistic, the budget insufficient, a site may have concerns, or a supporting department may not have the resources to fulfil their role in the trial.
Download a customisable feasibility and start up worksheet template here.
1.3 Conduct
The Conduct phase of the Clinical Trial Process, can be defined by the First Patient Visit (FPV) and Last Patient Visit (LPV) at your site, and can be divided into two further stages of:
a) Recruitment:
The recruitment period is the timeframe for all sites to enrol the participants, as outlined in the study protocol, to meet the objectives of the trial. This phase can also be called the 'accrual period'. A set timeframe for recruitment is usually defined globally, with minimum and maximum numbers to be recruited for each site defined in a site contract; however recruitment is globally competitive. This means that while a recruitment period is proposed, you may in fact have much less time if another country, or another site nationally, enrols participant quickly.
b) Maintenance:
This phase is defined by the end of recruitment and the last participant visit. All participants have been enrolled and you are now focusing on performing the trial visits, as required by the protocol. It's usually a time to allow reassessment of your resourcing to ensure the FTE support for the trial is adequate, and determine future trial needs for the unit.
1.4 Close-out
This is the final stage of the Clinical Trial Process and is marked by a site Close Out Visit (COV). This can occur from several months to a year after the final patient visit at your site and involves final review of data, regulatory binders and preparation for archiving of the study data. Archiving can only occur once the final study report, outlining the results of the trial have been provided to the trial site.
Download a customisable Archiving template here.
2. Commercially Sponsored vs Investigator Initiated Clinical Trials
|
Commercially Sponsored |
Investigator Initiated Clinical Trials |
|
It is initiated by a pharmaceutical/device company or other commercial entity and not by an investigator at an insured Victorian public health service. |
A pharmaceutical/device company is not acting as the Sponsor for the purposes of the CTN Scheme application. |
|
The Trial is conducted to investigate a drug/ device for commercial development by its manufacturer/sponsor. |
A pharmaceutical/device company is not directly funding the conduct of the study, that is, making payment to the relevant hospital or investigator. |
|
The protocol has been developed and is the responsibility of a pharmaceutical/device company or other commercial entity. |
The clinical trial addresses relevant clinical questions and not industry needs. |
|
The Principal Investigator or the Hospital/ Institution is the primary author and custodian of the clinical trial protocol. |
Other Considerations
There will be investigator initiated trials which will have some industry funding or industry contributions. Such funding arrangements must be declared in the protocol submission to ensure that the clinical trial retains its “investigator initiated” status.
Clinical trials that are initiated by co-operative/ collaborative research groups are treated as investigator initiated, provided they meet the requirements for co-operative studies.
Reference: VMIA Guidelines for Clinical Trials for Victorian Public Hospitals 2009
MACH and the VCCC Alliance have developed a suite of resources to support the conduct of Investigator Initiated Trials that can be accessed here.
3. New Starter Checklist
The New Starter Checklist has been developed to support the on boarding of new staff or those transitioning into a role in Clinical Research at Barwon Health. The checklist is provided to support your progress and will be used to record your competency in your new role. The checklist is in word format to allow you, your manager or coach to modify information for trial or protocol specific training.
Download the Barwon Health Clinical Researcher new starter checklist template here.
4. Clinical Trial Research Training
There are several elements of training that may be required for your role in Clinical Trial Research. These are summarised below.
4.1 International Conference on Harmonisation Good Clinical Practice Guidelines (ICH-GCP)
ICH-GCP or 'Good Clinical Practice' guidelines are the internationally accepted standard for designing, conducting, reporting, and recording of clinical trial research. GCP training is a mandatory requirement for anyone involved in clinical trial research and is available online via GROW here: Good Clinical Practice (GCP) Training
Course information:
This ICH E6 GCP training meets the minimum criteria for ICH GCP identified by TransCelerate BioPharma as necessary to enable mutual recognition of GCP training among trial sponsors. A certificate will be provided on completion of the training and is valid for 3 years. Please upload your certificate of completion on GROW.
When you are required to recertify in 3 years, a link to upload your certificate of completion will be provided.
Target Audience:
New and existing staff involved in conducting clinical trials at Barwon Health are required to complete ICH-GCP training.
New clinical trial and clinical research staff including Principal Investigators, Associate Investigators, CT Managers, CT/Research Coordinators, Research Assistants, HREC members and Research Development Unit Staff are required to complete the full A-CTEC GCP training.
Staff whose GCP training certificate is about to expire may complete a refresher course through Global Health Network.
The RDU may also ask existing researchers to complete A-CTEC GCP training via GROW as risk management following audits or incidents.
If GCP training does not appear as mandatory training within your GROW account, please ask your manager to add you to the researcher list in GROW.
Duration: A-CTEC = 6-8 hours. GHN = Refresher 1 hour
Face to Face training is also available, at cost, through external providers which provide details on applying the practicalities of GCP in the context of a trial. External Training providers used by Units at Barwon Health are below:
|
Training Provider |
Website |
|
ARCS Australia |
|
|
Datapharm Australia |
|
|
Praxis |
4.2 International Air Transport Association (IATA) Dangerous Goods Training
IATA Dangerous Good training is required if you are a shipper of Dangerous Goods by Air for any of your projects. Training is provided by the Civil Aviation Academy of Australasia (CAAA) in various format. More information can be found on the CAAA website, or you can contact them by telephone on (08) 6180 7939.
Last Modified: Monday, 26 June 2023
